What Is the Most Reactive Family on the Periodic Table
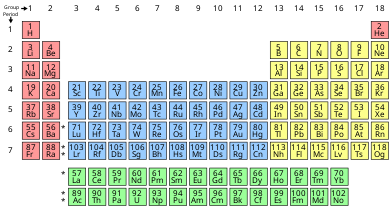
In the periodic table of the elements, each numbered column is a group.
In chemistry, a group (also known as a family [1]) is a column of elements in the periodic table of the chemic elements. In that location are 18 numbered groups in the periodic table; the f-block columns (between groups two and three) are not numbered. The elements in a group have like concrete or chemic characteristics of the outermost electron shells of their atoms (i.east., the same core charge), considering nearly chemical properties are dominated by the orbital location of the outermost electron.
There are three systems of group numbering for the groups; the same number may exist assigned to unlike groups depending on the system being used. The mod numbering system of "group one" to "grouping 18" has been recommended by the International Matrimony of Pure and Practical Chemistry (IUPAC) since nearly 1990. It replaces 2 older incompatible naming schemes, used by the Chemical Abstract Service (CAS, more than pop in the US), and past IUPAC before 1990 (more popular in Europe). The system of xviii groups is generally accepted by the chemistry community, simply some dissent exists most membership of several elements. Disagreements mostly involve elements number one and ii (hydrogen and helium), too every bit inner transition metals.
Groups may likewise be identified using their topmost chemical element, or take a specific proper noun. For instance, group 16 is too described as the "oxygen group" and as the "chalcogens". An exception is the "iron grouping", which usually refers to "group eight", but in chemistry may also mean iron, cobalt, and nickel, or another set of elements with similar chemical properties. In astrophysics and nuclear physics, it unremarkably refers to iron, cobalt, nickel, chromium, and manganese.
Group names [edit]
In history, several sets of group names have been used:[two] [3]
| IUPAC group | anea | 2 | n/a | threeb | iv | 5 | half-dozen | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | xv | sixteen | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mendeleev (I–Viii) | IA | IIA | ThreeB | 4B | VB | VIB | SevenB | VIIIB | IB | IIB | IIIB | 4B | VB | VIB | SevenB | c | |||
| CAS (US, A-B-A) | IA | IIA | IIIB | IVB | VB | VIB | VIIB | VIIIB | IB | IIB | IIIA | IVA | VA | VIA | VIIA | VIIIA | |||
| old IUPAC (Europe, A-B) | IA | IIA | IIIA | IVA | VA | VIA | VIIA | VIIIB | IB | IIB | IIIB | IVB | VB | VIB | VIIB | 0 | |||
| Fiddling name | H and Alkali metalsr | Alkaline earth metalsr | Coinhistoric period metalsd | Triels | Tetrels | Pnictogensr | Chalcogensr | Halogensr | Noble gasesr | ||||||||||
| Name by elementr | Lithium group | Beryllium group | Scandium group | Titanium grouping | Vanadium grouping | Chromium group | Manganese group | Iron group | Cobalt grouping | Nickel grouping | Copper grouping | Zinc group | Boron grouping | Autobon grouping | Nitrogen group | Oxygen group | Fluorine group | Helium or Neon grouping | |
| Period one | H | He | |||||||||||||||||
| Period 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||
| Flow 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| Period 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Menstruation 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Catamenia six | Cs | Ba | La–Yb | Lu | Hf | Ta | Westward | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Period seven | Fr | Ra | Ac–No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
a Group 1 is composed of hydrogen (H) and the brine metals. Elements of the group accept one s-electron in the outer electron beat out. Hydrogen is non considered to be an alkaline as it is non a metal, though information technology is more coordinating to them than whatsoever other grouping. This makes the group somewhat exceptional.
n/a Do non have a grouping number
b The composition of group 3 is not agreed among sources: run into Periodic tabular array#Group 3 and Group 3 element#Dispute on limerick. General inorganic chemical science texts ordinarily put scandium (Sc), yttrium (Y), lanthanum (La), and actinium (Air conditioning) in group 3, and so that Ce–Lu and Th–Lr become the f-block between groups three and 4. However, sources that written report the affair usually put scandium, yttrium, lutetium (Lu), and lawrencium (Lr) in group 3, equally shown here. Some sources, including IUPAC, currently follow a compromise that puts La–Lu and Ac–Lr as the f-block rows, leaving the heavier members of group 3 ambiguous. The system with Sc, Y, Lu, and Lr in group 3 has been recommended by a 2021 IUPAC preliminary report on this question.
c Group 18, the noble gases, were non discovered at the time of Mendeleev'due south original table. After (1902), Mendeleev accustomed the bear witness for their existence, and they could be placed in a new "group 0", consistently and without breaking the periodic table principle.
d Authors differ on whether roentgenium (Rg) is considered a coinage metal. Information technology is in group 11, like the other coinage metals, and is expected to exist chemically similar to golden.[iv] On the other hand, beingness extremely radioactive and short-lived, it cannot actually be used for coinage as the name suggests, and on that basis it is sometimes excluded.[5]
r Group name as recommended past IUPAC.
| New IUPAC proper name | Onetime IUPAC (Europe) | CAS proper name (U.S.) | Proper noun by element | IUPAC recommended petty proper noun | Other petty proper name |
|---|---|---|---|---|---|
| Group 1 | IA | IA | lithium family | hydrogen and alkali metals* | |
| Group ii | IIA | IIA | glucinium family | element of group i earth metals* | |
| Group 3 | IIIA | IIIB | scandium family unit | ||
| Group 4 | IVA | IVB | titanium family unit | ||
| Group five | VA | VB | vanadium family | ||
| Grouping six | VIA | VIB | chromium family | ||
| Group seven | VIIA | VIIB | manganese family | ||
| Group viii | Viii | VIIIB | iron family | ||
| Group 9 | 8 | VIIIB | cobalt family unit | ||
| Grouping 10 | VIII | VIIIB | nickel family | ||
| Group 11 | IB | IB | copper family | coinage metals | |
| Group 12 | IIB | IIB | zinc family unit | ||
| Group xiii | IIIB | IIIA | boron family | triels from Greek tri (three, Three)[6] [7] | |
| Group 14 | IVB | IVA | carbon family | tetrels from Greek tetra (four, Iv)[6] [7] | |
| Group fifteen | VB | VA | nitrogen family | pnictogens* | pentels from Greek penta (five, V)[seven] |
| Grouping sixteen | VIB | VIA | oxygen family unit | chalcogens* | |
| Group 17 | VIIB | VIIA | fluorine family | halogens* | |
| Group eighteen | 0 | VIIIA | helium family or neon family | noble gases* |
Some other names take been proposed and used without gaining wide acceptance:
- "volatile metals" for group 12;[8]
- "icosagens" for group 13;[nine]
- "crystallogens",[6] "adamantogens",[10] and "merylides"[ commendation needed ] for grouping 14;
- "aerogens" for group 18.[7]
CAS and one-time IUPAC numbering (A/B) [edit]
Ii earlier group number systems exist: CAS (Chemical Abstracts Service) and erstwhile IUPAC. Both use numerals (Arabic or Roman) and messages A and B. Both systems concur on the numbers. The numbers point approximately the highest oxidation number of the elements in that group, and so indicate similar chemistry with other elements with the aforementioned numeral. The number proceeds in a linearly increasing way for the most function, one time on the left of the table, and once on the correct (see List of oxidation states of the elements), with some irregularities in the transition metals. Nonetheless, the two systems utilize the letters differently. For example, potassium (M) has one valence electron. Therefore, it is located in group 1. Calcium (Ca) is in group 2, for it contains 2 valence electrons.
In the old IUPAC system the letters A and B were designated to the left (A) and right (B) part of the table, while in the CAS organisation the messages A and B are designated to main group elements (A) and transition elements (B). The old IUPAC system was frequently used in Europe, while the CAS is most common in America. The new IUPAC scheme was adult to supersede both systems as they confusingly used the same names to hateful different things. The new system simply numbers the groups increasingly from left to correct on the standard periodic table. The IUPAC proposal was offset circulated in 1985 for public comments,[2] and was after included equally part of the 1990 edition of the Classification of Inorganic Chemistry.[eleven]
See also [edit]
- Period (periodic table)
References [edit]
- ^ "The Periodic Table Terms". www.shmoop.com . Retrieved 2018-09-xv .
- ^ a b Fluck, E. (1988). "New Notations in the Periodic Table" (PDF). Pure Appl. Chem. IUPAC. 60 (3): 431–436. doi:ten.1351/pac198860030431. S2CID 96704008. Retrieved 24 March 2012.
- ^ IUPAC (2005). "Nomenclature of inorganic chemistry" (PDF).
- ^ Conradie, Jeanet; Ghosh, Abhik (2019). "Theoretical Search for the Highest Valence States of the Coinage Metals: Roentgenium Heptafluoride May Exist". Inorganic Chemistry. 58 (13): 8735–8738. doi:10.1021/acs.inorgchem.9b01139.
- ^ Grochala, Wojciech; Mazej, Zoran (2015). "Chemistry of silver(2): a cornucopia of peculiarities". Philosophical Transactions of the Imperial Guild A. 373. doi:10.1098/rsta.2014.0179. Retrieved 23 December 2021.
- ^ a b c Liu, Ning; Lu, Na; Su, Yan; Wang, Pu; Quan, Xie (2019). "Fabrication of g-C3Niv/Ti3C2 blended and its visible-low-cal photocatalytic capability for ciprofloxacin degradation". Separation and Purification Technology. 211: 782–789. doi:10.1016/j.seppur.2018.10.027. S2CID 104746665. Retrieved 17 August 2019.
- ^ a b c d Rich, Ronald (2007). Inorganic Reactions in H2o. Springer. pp. 307, 327, 363, 475. doi:10.1007/978-3-540-73962-three. ISBN9783540739616.
- ^ "volatile metal". Glosbe . Retrieved xiv Jan 2021.
{{cite spider web}}: CS1 maint: url-condition (link) - ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2d ed.). Butterworth-Heinemann. p. 227. ISBN978-0-08-037941-8.
- ^ William B. Jensen, The Periodic Law and Tabular array
- ^ Leigh, G. J. Classification of Inorganic Chemical science: Recommendations 1990. Blackwell Science, 1990. ISBN 0-632-02494-one.
Farther reading [edit]
- Scerri, E. R. (2007). The periodic table, its story and its significance . Oxford University Press. ISBN978-0-xix-530573-nine.
Source: https://en.wikipedia.org/wiki/Group_(periodic_table)
0 Response to "What Is the Most Reactive Family on the Periodic Table"
Post a Comment